RESEARCH OVERVIEW
Our studies focus on four major areas:
Axon Degeneration: Defining Mechanisms and Developing Therapies
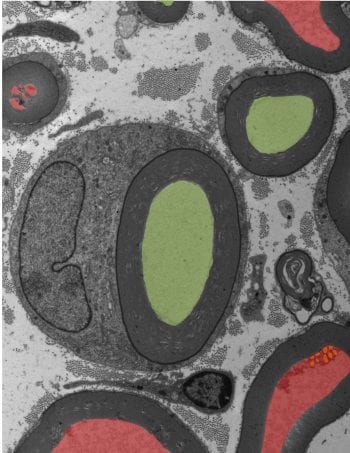
Profiles of myelin-wrapped axons are analyzed in studies of axonal survival in mouse models of peripheral neuropathy, ALS, and glaucoma.
Axon loss drives morbidity and progression of many common neurological diseases including peripheral neuropathy, glaucoma, traumatic brain injury, multiple sclerosis, and neurodegenerative diseases such as Alzheimer’s Disease, Parkinson’s Disease, and ALS.
Axon degeneration is a genetically-encoded program of subcellular self-destruction. We use Drosophila, mice, and human iPSC-derived neurons to define the molecular mechanisms of the axon degeneration pathway.
We identified SARM1 as the central executioner of the axonal degeneration program and demonstrated that it is the founding member of an ancient class of enzymes that cleave the essential metabolite NAD.
Using these mechanistic insights, we are now developing small molecule inhibitors and gene therapy approaches to block the SARM1 pathway as potential therapies for neurodegenerative disease.
This work has been done in close collaboration with the lab of Dr. Jeffrey Milbrandt.
Axon Regeneration
Neurons in the peripheral nervous system can regenerate axons lost to injury or disease, but neurons in the central nervous system fail to regenerate injured axons. We discovered that the neuronal stress kinase DLK is required for axon regeneration, activating a pro-regenerative gene expression program that promotes neuronal repair. Interestingly, DLK can also promote axon degeneration and neuronal cell death. We are dissecting these disparate functions of DLK to define how neuronal stress can trigger either regenerative or degenerative responses.
In addition, we use high-content automated screening approaches to undertake large-scale drug and genetic screens to identify novel therapeutic candidates that promote axonal regeneration and inhibit axonal degeneration and neuronal cell death.
Neuroimmunology

TIR domain proteins are an ancient family of NAD cleaving enzymes important for the intersection of metabolism, inflammation, and neurodegeneration.
The TIR domain is the canonical protein motif of innate immune signaling, previously defined only as a scaffold for organizing signal transduction cascades. Through our studies of SARM1, we discovered that the TIR domain is an enzyme that cleaves the essential metabolic co-factor NAD. We discovered that this ancient function of TIR domains is conserved in animals, plants, bacteria, and archaebacteria, thereby redefining our understanding of innate immune signaling across the domains of life.
We are exploring the role of TIR domain enzymes as central nodes for the integration of immune signaling, metabolism, and inflammation in neurodegenerative disease.
Synaptic and Axonal Physiology

The Drosophila neuromuscular junction is a powerful model system for studies of synaptic and axonal physiology.
We explore mechanisms controlling synaptic strength and axonal excitability.
Using Drosophila and mouse models of Fragile X syndrome, we are defining key functional targets of FMRP in hopes of developing tools to improving synaptic function in this disorder.
We also investigate the role of glial cells in maintaining healthy and functional axons, defining key pathways in the glia that promote proper axonal physiology.
Lab News
Dr. O’Connor Wins the 2023 Larry Sandler Award
Aaron wins Neuroscience Community Award for Excellence in Mentoring
Key finding supporting role of STMN2 in ALS

A Productive Collaboration
Much of the work by the DiAntonio Lab has been done in a close and productive collaboration with the Milbrandt Lab. In 2015, construction of the Couch Biomedical Research Building, pictured above, enabled the two labs to move into a shared space, facilitating even greater interaction. In 2019, Dr. DiAntonio and Dr. Milbrandt were named Co-Directors of the Needleman Center for Neurometabolism and Axonal Therapeutics.
